Synthetic diamonds are created using two processes: chemical vapor deposition (CVD) and high-pressure, high temperature (HPHT). It’s important to understand how these processes affect diamond quality. This article reviews the main mistakes to avoid when buying a lab-created diamond:
- CVD – Avoid brown color hue. Avoid diamonds with strain lines that cause them to look fuzzy.
- HPHT – Avoid blue color hue (“blue nuance” or “faint blue”).
There’s a lot of variation in quality between CVD and HPHT diamond manufacturers. We recommend going up in color to avoid brown CVD and blue HPHT hues.
We prefer HPHT diamonds over CVD diamonds due to the lack of fuzzy strain-induced birefringence. It’s one less thing to worry about when choosing a lab diamond. In our lab diamond price curves, we pre-screen for non-blue diamonds using computer vision to make the search easier.
CVD Brown Color Hue
CVD Brown Hue TL;DR – Vacancy defects that cause brown color in CVD diamonds adversely affect the optical properties of the material. Natural brown diamonds are not recommended; avoid brown lab diamonds too!
Many CVD diamonds have a brown hue as described in GIA’s CVD diamond review:
A slower growth process generally results in colorless crystals of very high purity and low defect content; however, the growth chemistry of many mass-produced CVD synthetics is manipulated by adding nitrogen or oxygen to the gas mixture to improve crystal quality (Liang et al., 2009), or they are treated at HPHT conditions afterward to remove any brown coloration.
Eaton-Magana, S., & Shigley, J. E. (2016). OBSERVATIONS ON CVD-GROWN SYNTHETIC DIAMONDS: A REVIEW. Gems & Gemology, 52(3).

Although the source of brown color in CVD diamonds remains an open research question, vacancy (nitrogen-vacancy-hydrogen [NVH]) is one known cause.
During high growth rate CVD processes, the growing surface can become rough and stepped and then rapidly covered to leave small voids or clusters of vacancies. The high growth temperature would allow the incorporated vacancies to become mobile, re-organizing by dissolution into vacancy disks, with a consequent energy reduction.
Hounsome, L. S., Jones, R., Martineau, P. M., Fisher, D., Shaw, M. J., Briddon, P. R., & Öberg, S. (2006). Origin of brown coloration in diamond. Physical Review B, 73(12), 125203.
Defects causing colour in nitrogen-doped chemical vapour-deposited (CVD) diamond can adversely affect the exceptional optical, electronic and spintronic properties of the material.
In CVD diamond, the defects responsible for the brown colour are different to those present in natural diamonds and are removed at a lower treatment temperature. The colour is attributed to several features in the absorption spectrum. One, the 520 nm (2.39 eV) band, is ascribed to the NVH 0 defect. It is nominally midgap (>2 eV from either band edge). It is removed after treatment at 1800 ◦ C. NVH 0 is highly charge transfer active, increasing in intensity upon exposure to UV light and decreasing with heating, due to the gain/loss of electrons between it and N S defects already present in the material. Two other absorption features, namely a gradual absorption ‘profile’ that results in the brown colour, and a band centred at 360 nm (3.49 eV), appear to be correlated and relate to a defect that is distinct from NVH 0 . This defect also happens to be highly charge transfer active and increases/reduces in intensity with UV radiation/heat in a similar manner to NVH 0 .
Khan, R. U. A., Cann, B. L., Martineau, P. M., Samartseva, J., Freeth, J. J. P., Sibley, S. J., … & Twitchen, D. J. (2013). Colour-causing defects and their related optoelectronic transitions in single crystal CVD diamond. Journal of Physics: Condensed Matter, 25(27), 275801.
Brown hue is readily apparent when viewing a diamond in profile view. Compare the two H color lab-created diamonds below. The 2.03 H VS1 CVD diamond is brown. The 1.24 H VVS1 diamond is yellow, similar to a natural diamond yellow hue.
Brown hue is possible for all color grades since it’s inherent to the CVD process. Either go up in color to D/E to minimize the appearance or avoid it entirely. If you click on the brown H, you’ll see faint strain lines that you should also avoid (discussed in next section).


CVD Strain Induced Birefringence (Causing a Fuzzy Appearance)
CVD Strain Induced Birefringence and Fuzzy Appearance TL;DR – CVD diamonds are grown layer by layer. Mismatches in the crystal lattice between layers introduce anomalous birefringence, which causes the diamond to look fuzzy. Avoid fuzzy CVD diamonds with visible strain lines!
A perfect diamond is isotropic (uniform crystal in all directions, singly refractive). It won’t exhibit birefringence, which is what causes doubly refractive gemstones (e.g. moissanite) to look fuzzy. Below is a description from a reference book.
The pre-eminence of diamond as a gem-stone is due to a combination of optical and mechanical properties. The important optical properties are its high refractive index, its zero birefringence (if unstressed) and its dispersion. These give diamond its so-called “brilliance”, “sparkliness”, and “fire”.
Many minerals exhibit double-refraction with refracted light split into the ordinary and extraordinary rays; birefringence is a measure of the difference between the two refractive indices. Double-refraction is a disadvantage with a gem-stone since it gives a fuzzy appearance due to the eye seeing two images of the back surface. Since diamond is cubic, and so optically isotropic, it does not suffer from this disadvantage.
Diamond and Diamond-like Films and Coatings. (2012). United States: Springer US.
However, a less than perfect diamond can exhibit birefringence. The excerpt below is from a paper by Sir CV Raman, the famous Indian physicist who won the Nobel prize in physics for the Raman effect. Even in the 1930s, it was observed that very clear diamonds show little or no double refraction and that “flawed diamonds, brownish tinted crystals, and distorted crystals” show birefringence. Perfection of external form and optical quality should show the optical isotropy characteristic of a diamond. Nobel-prize winning optical physicists were advising against “brownish tinted crystals and distorted crystals” back in the 1940s!
Mineralogists have long been familiar with the fact that diamonds exhibit a very varied behaviour when examined under the polarising microscope. Sinor (1930) made extensive observations on the diamonds mined in the Panna State and found that very clear crystals in which all the faces were symmetrically developed showed very little or no double refraction. On the other hand, crystals containing flaws and inclusions, distorted crystals, and brownish tinted crystals showed bands and stripes of colour between crossed nicols. Since perfection of external form and of optical quality are both indicative of a homogeneity of structure, the specimens exhibiting them should naturally also show the optical isotropy characteristic of a cubic crystal.
Raman, C. V., & Rendall, G. R. (1944, May). Birefringence patterns in diamond. In Proceedings of the Indian Academy of Sciences-Section A (Vol. 19, No. 5, p. 265). Springer India.
CVD diamonds often exhibit anomalous birefringence due to the way they’re grown layer by layer.
Single crystal diamond grown by chemical vapour deposition (CVD) often exhibits strain induced birefringence arising from bundles of edge dislocations lying almost parallel to the [001] growth axis.
Pinto, H., & Jones, R. (2009). Theory of the birefringence due to dislocations in single crystal CVD diamond. Journal of Physics: Condensed Matter, 21(36), 364220.
Birefringence in diamond is an anomalous optical property. Its occurrence is the result of strain in the diamond lattice, modifying its natural isotropic properties.
The strain is very localized about the interface between the two layers because the lattice mismatch is so small. This phenomenon has been observed for cross-sectional slices through chemical-vapour-deposition (CVD) synthetic diamond.
Daniel Howell; Strain-induced birefringence in natural diamond: a review. European Journal of Mineralogy ; 24 (4): 575–585. doi: https://doi.org/10.1127/0935-1221/2012/0024-2205
It’s long been known that dislocations create whitish graining in natural diamonds.
Dislocations in the diamond lattice are the main cause of banded or tatami graining. Lattice distortion affects many physical properties, such as refractive index. Instead of light following its typical path through the diamond, in the distorted region it is refracted or diffused due to the difference in R. I. between that region and the surrounding lattice.
In extreme cases, the graining is so intense and widespread that the observer has the sensation of looking through a screen (i.e., the “crisp” appearance is diminished.)
King, J. M., Moses, T. M., & Wang, W. (2006). The impact of internal whitish and reflective graining on the clarity grading of D-to-Z color diamonds at the GIA laboratory. Gems and Gemology, 42(4), 206.

A GIA review shows what this CVD strain looks like under cross-polarizers.
The lack of observable strain (anomalous bi-refringence) when a diamond is viewed with polarized light does provide a strong indication of HPHT growth (figure A-1). Natural diamonds are typically subjected to varying stresses during their long growth and transport history. In contrast, HPHT synthetic diamonds are grown in a uniform high-pressure field.
Eaton-Magaña, S., Shigley, J. E., & Breeding, C. M. (2017). OBSERVATIONS ON HPHT-GROWN SYNTHETIC DIAMONDS: A REVIEW. Gems & Gemology, 53(3).

You don’t need cross-polarizers to see strain lines. Similar to the photomicrographs of whitish graining in natural diamonds, strain lines are visible in high quality photos, such as in the 2.16ct and 1.72ct G VS1’s below. Strain lines are not evaluated as part of clarity grading, which is why these diamonds can still receive a VS1 clarity grade despite the fuzziness. Heed the lessons from natural diamonds, don’t buy fuzzy brown CVD diamonds with visible strain lines!


In contrast, HPHT diamonds do not exhibit such strain lines. Note how much clearer a diamond without strain lines looks compared to the CVD examples above.

HPHT Blue Color Hue
HPHT Blue Hue TL;DR – Boron impurities in HPHT diamonds cause a “blue nuance.” Avoid blue lab diamonds!
The HPHT process is different from the CVD process. HPHT diamonds don’t exhibit brown color and they’re not fuzzy because they don’t have strain-induced birefringence. Instead, HPHT diamonds have a different issue: boron impurities that create a blue hue.
Blue HPHT synthetics (along with a majority of colorless HPHT samples) were type IIb, indicating either intentional doping or accidental contamination with boron in the growth chamber. Figure 15 shows the distribution of diamond types among HPHT synthetics based on bodycolor. Those with yellow color were predominantly type Ib or a combination of type Ib and type IaA. The blue synthetics were exclusively type IIb. Colorless HPHT synthetics were type IIa or, more commonly, type IIb (where the amount of boron was insufficient to produce any blue coloration). The post-growth treated pinks were type IIa or type Ib, but with very low nitrogen concentrations.
Eaton-Magaña, S., Shigley, J. E., & Breeding, C. M. (2017). OBSERVATIONS ON HPHT-GROWN SYNTHETIC DIAMONDS: A REVIEW. Gems & Gemology, 53(3).
Natural type IIb diamonds with a blue hue are extremely rare. A blue tint strongly suggests that it’s lab-created.
Blue hue is readily apparent when viewing a diamond in profile view. The 1.03 H VS1 HPHT diamond is blue. The 1.24 H VVS1 is yellow, similar to a natural diamond yellow hue. You can also avoid the blue hue by going higher in color to D/E, such as with the 2.00 E VS1. The brown CVD 2.03 H VS1 is also shown below to facilitate the full side-by-side color comparison. Which diamond would you prefer?




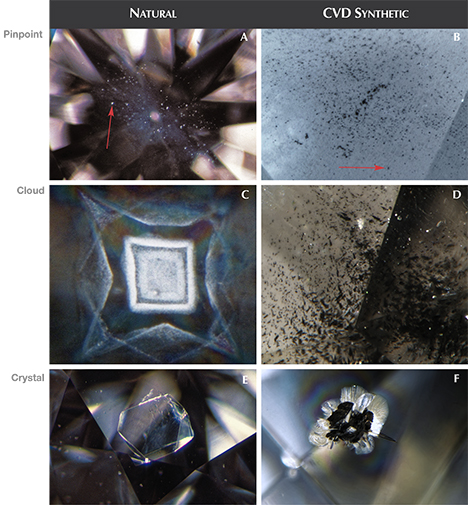
Inclusions
CVD diamonds typically have black graphitic inclusions while HPHT diamonds typically have black flux inclusions. Although the inclusion material is different for the two processes, it doesn’t make any practical difference when choosing a diamond. In both cases, you’re trying to find a diamond that’s clean to the naked eye.
Inclusions should dispel the notion that lab diamonds are more perfect that natural diamonds.
Conclusion
We’ve highlighted the main quality issues to look out for with lab-created diamonds. Hopefully this helps you pick a nice one!